Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
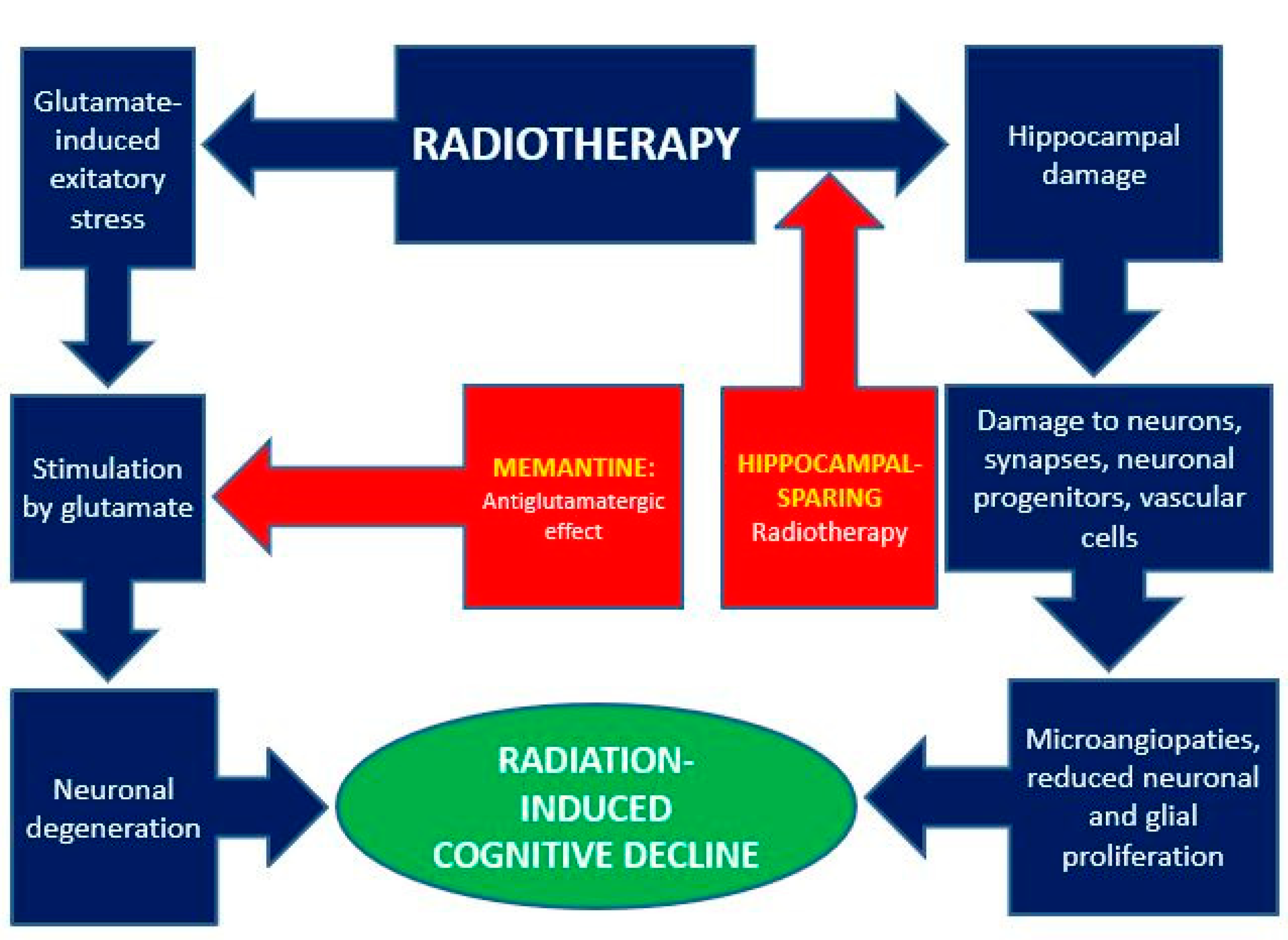
3.1. Memantine and Radiation-Induced Effects on the Central Nervous System
3.1.1. Neuronal Degeneration and Memantine
3.1.2. Radiation-Induced Brain Toxicity
3.1.3. Cognitive Decline (CD)
3.1.4. Radiation-Induced Cognitive Decline (RICD)
3.2. Main Evidence
3.2.1. RTOG 0614 Study
3.2.2. RTOG 0614 Trial-Related Studies
3.2.3. Hippocampal-Avoidance: The RTOG 0933 Trial
3.2.4. Pharmacological Prophylaxis plus Anatomical Sparing: The NRGCC001 Trial
3.2.5. Scientific Community Reactions
3.2.6. State of Art
3.3. New Scenarios
The Antitumor Effect of Memantine
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chao, J.H.; Phillips, R.; Nickson, J.J. Roentgen-ray therapy of cerebral metastases. Cancer 1954, 7, 682–689. [Google Scholar] [CrossRef]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patchell, R.A.; Tibbs, P.A.; Regine, W.F.; Dempsey, R.J.; Mohiuddin, M.; Kryscio, R.J.; Markesbery, W.R.; Foon, K.A.; Young, B. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA 1998, 280, 1485–1489. [Google Scholar] [CrossRef]
- Salzmann, M.; Hess, K.; Lang, K.; Enk, A.H.; Jordan, B.; Hassel, J.C. Long-term neurocognitive function after whole-brain radiotherapy in patients with melanoma brain metastases in the era of immunotherapy. Strahlenther Onkol. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Surendran, H.P.; Narmadha, M.P.; Kalavagunta, S.; Sasidharan, A.; Dutta, D. Preservation of cognitive function after brain irradiation. J. Oncol. Pharm Pract. 2022, 10781552221077037. [Google Scholar] [CrossRef]
- Robin, T.P.; Rusthoven, C.G. Strategies to Preserve Cognition in Patients with Brain Metastases: A Review. Front Oncol. 2018, 8, 415. [Google Scholar] [CrossRef]
- Parsons, M.W.; Peters, K.B.; Floyd, S.R.; Brown, P.; Wefel, J.S. Preservation of neurocognitive function in the treatment of brain metastases. NeuroOncol. Adv. 2021, 3 (Suppl. 5), v96–v107. [Google Scholar] [CrossRef]
- Orrego, F.; Villanueva, S. The chemical nature of the main central excitatory transmitter: A critical appraisal based upon release studies and synaptic vesicle localization. Neuroscience 1993, 56, 539–555. [Google Scholar] [CrossRef]
- Willard, S.S.; Koochekpour, S. Glutamate, glutamate receptors, and downstream signaling pathways. Int. J. Biol. Sci. 2013, 9, 948–959. [Google Scholar] [CrossRef] [Green Version]
- Chaffey, H.; Chazot, P. NMDA receptor subtypes: Structure, function and therapeutics. Curr. Anaesth. Crit. Care 2008, 19, 183–201. [Google Scholar] [CrossRef]
- Lee, M.C.; Ting, K.K.; Adams, S.; Brew, B.J.; Chung, R.; Guillemin, G.J. Characterisation of the expression of NMDA receptors in human astrocytes. PLoS ONE 2010, 5, e14123. [Google Scholar] [CrossRef] [PubMed]
- Conti, F.; Debiasi, S.; Minelli, A.; Melone, M. Expression of NR1 and NR2A/B subunits of the NMDA receptor in cortical astrocytes. Glia 1996, 17, 254–258. [Google Scholar] [CrossRef]
- MacDonald, J.F.; Jackson, M.F.; Beazely, M.A. Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors. Crit. Rev. Neurobiol. 2006, 18, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Parsons, C.G.; Karcz-Kubicha, M.; Schwaier, A.; Popik, P.; Wedzony, K.; Lazarewicz, J.; Quack, G. GlycineB antagonists as potential therapeutic agents. Previous hopes and present reality. Amino Acids 1998, 14, 235–239. [Google Scholar] [CrossRef]
- Hansen, K.B.; Yi, F.; Perszyk, R.E.; Menniti, F.S.; Traynelis, S.F. NMDA receptors in the central nervous system. Methods Mol. Biol. 2017, 1677, 1–80. [Google Scholar]
- Yohay, K.; Tyler, B.; Weaver, K.D.; Pardo, A.C.; Gincel, D.; Blakeley, J.; Brem, H.; Rothstein, J.D. Efficacy of local polymer-based and systemic delivery of the antiglutamatergic agents riluzole and memantine in rat glioma models. J. Neurosurg. 2014, 120, 854–863. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S. Memantine: Pharmacologial properties and clinical uses. Neurol. India 2004, 52, 307–309. [Google Scholar]
- Ye, Z.C.; Sontheimer, H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999, 59, 4383–4391. [Google Scholar]
- Chen, H.S.; Lipton, S.A. The chemical biology of clinically tolerated NMDA receptor antagonists. J. Neurochem. 2006, 97, 1611–1626. [Google Scholar] [CrossRef]
- Glezer, I.; Zekki, H.; Scavone, C.; Rivest, S. Modulation of the innate immune response by NMDA receptors has neuropathological consequences. J. Neurosci. 2004, 23, 11094–11103. [Google Scholar] [CrossRef]
- Perez-Otano, I.; Larsen, R.S.; Wesseling, J.F. Emerging roles of glun3-containing NMDA receptors in the CNS. Nat. Rev. Neurosci. 2016, 17, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Olivares, D.; Deshpande, V.K.; Shi, Y.; Lahiri, D.K.; Greig, N.H.; Rogers, J.T.; Huang, X. N-methyl D-aspartate (NMDA) receptor antagonists and memantine treatment for Alzheimer’s disease, vascular dementia and Parkinson’s disease. Curr. Alzheimer Res. 2012, 9, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Silvente, L.; Capella, D.; Garre-Olmo, J.; Vilalta-Franch, J.; Castells, X. Predictors of discontinuation, efficacy, and safety of memantine treatment for Alzheimer’s disease: Meta-analysis and meta-regression of 18 randomized clinical trials involving 5004 patients. BMC Geriatr. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, S.J.; Grossberg, G.T. Memantine: A review of studies into its safety and efficacy in treating Alzheimer’s disease and other dementias. Clin. Interv. Aging 2009, 4, 367–377. [Google Scholar] [PubMed] [Green Version]
- Parsons, C.G.; Danysz, W.; Quack, G. Memantine is a clinically well tolerated N-methyl-daspartate (NMDA) receptor antagonist—a review of preclinical data. Neuropharmacology 1999, 38, 735–767. [Google Scholar] [CrossRef]
- Peeters, M.; Page, G.; Maloteaux, J.M.; Hermans, E. Hypersensitivity of dopamine transmission in the rat striatum after treatment with the NMDA receptor antagonist amantadine. Brain Res. 2002, 949, 32–41. [Google Scholar] [CrossRef]
- Lannes, B.; Micheletti, G. Sensitization of the striatal dopaminergic system induced by chronic administration of a glutamate antagonist in the rat. Neurosci. Biobehav. Rev. 1997, 21, 417–424. [Google Scholar] [CrossRef]
- Gash, D.M.; Zhang, Z.; Gerhardt, G. Neuroprotective and neurorestorative properties of GDNF. Ann. Neurol. 1998, 44, S121–S125. [Google Scholar] [CrossRef]
- Caumont, A.S.; Octave, J.N.; Hermans, E. Amantadine and memantine induce the expression of the glial cell line-derived neurotrophic factor in C6 glioma cells. Neurosci Lett. 2006, 394, 196–201. [Google Scholar] [CrossRef]
- Chen, H.S.; Lipton, S.A. Mechanism of memantine block of NMDA-activated channels in rat retinal ganglion cells: Uncompetitive antagonism. J. Physiol. 1997, 499, 27–46. [Google Scholar] [CrossRef]
- Chen, H.S.; Pellegrini, J.W.; Aggarwal, S.K.; Lei, S.Z.; Warach, S.; Jensen, F.E.; Lipton, S.A. Open-channel block of N-methyl-D-aspartate (NMDA) responses by memantine: Therapeutic advantage against NMDA receptor-mediated neurotoxicity. J. Neurosci. 1992, 12, 4427–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, J.W.; Lipton, S.A. Delayed administration of memantine prevents N-methyl-Daspartate receptor-mediated neurotoxicity. Ann. Neurol 1993, 33, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Tzeng, N.S.; Qian, L.; Wei, S.J.; Hu, X.; Chen, S.H.; Rawls, S.M.; Flood, P.; Hong, J.S.; Lu, R.B. Novel neuroprotective mechanisms of memantine: Increase in neurotrophic factor release from astroglia and anti-inflammation by preventing microglial activation. Neuropsychopharmacology 2009, 34, 2344–2357. [Google Scholar] [CrossRef]
- Stieg, P.E.; Sathi, S.; Warach, S.; Le, D.A.; Lipton, S.A. Neuroprotection by the NMDA receptor-associated open-channel blocker memantine in a photothrombotic model of cerebral focal ischemia in neonatal rat. Eur. J. Pharmacol. 1999, 375, 115–120. [Google Scholar] [CrossRef]
- Sinn, D.I.; Lee, S.T.; Chu, K.; Jung, K.H.; Song, E.C.; Kim, J.M.; Park, D.K.; Kim, M.; Roh, J.K. Combined neuroprotective effects of celecoxib and memantine in experimental intracerebral hemorrhage. Neurosci. Lett. 2007, 411, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Qiu, J.; Alcon, S.; Hashim, J.; Rotenberg, A.; Sun, Y.; Meehan, W.P., 3rd; Mannix, R. Memantine improves outcomes after repetitive traumatic brain injury. Behav. Brain Res. 2018, 340, 195–204. [Google Scholar] [CrossRef]
- Schneider, E.; Fischer, P.A.; Clemens, R.; Balzereit, F.; Funfgeld, E.W.; Haase, H.J. Effects of oral memantine administration on parkison symptoms. Results of a placebo controlled multicenter study. Dtsch. Med. Wochenschr. 1984, 109, 987–990. [Google Scholar] [CrossRef]
- Kornhuber, J.; Weller, M.; Schoppmeyer, K.; Riederer, P. Amantadine and memantine are NMDA receptor antagonists with neuroprotective properties. J. Neural Transm. Suppl 1994, 43, 91–104. [Google Scholar]
- Parsons, C.G.; Quack, G.; Bresink, I.; Baran, L.; Przegalinski, E.; Kostowski, W.; Krzascik, P.; Hartmann, S.; Danysz, W. Comparison of the potency, kinetics and voltage-dependency of a series of uncompetitive NMDA receptor antagonists in vitro with anticonvulsive and motor impairment activity in vivo. Neuropharmacology 1995, 34, 1239–1258. [Google Scholar] [CrossRef]
- Wenk, G.; Danysz, W.; Mobley, S.L. Mk-801, memantine and amantadine show neuroprotective activity in the nucleus basalis magnocellularis. Eur. J. Pharmacol. 1995, 293, 267–270. [Google Scholar] [CrossRef]
- Jain, K.K. Evaluation of memantine for neuroprotection in dementia. Expert Opin. Investig. Drugs 2000, 9, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Danysz, W.; Parsons, C.G.; Kornhuber, J.; Schmidt, W.J.; Quack, G. Aminoadamantanes as NMDA receptor antagonists and antiparkinsonian agents—preclinical studies. Neurosci. Biobehav. Rev. 1997, 21, 455–468. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J.; Memantine Study Group. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef] [PubMed]
- Emre, M.; Tsolaki, M.; Bonuccelli, U.; Destée, A.; Tolosa, E.; Kutzelnigg, A.; Ceballos-Baumann, A.; Zdravkovic, S.; Bladström, A.; Jones, R.; et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010, 9, 969–977. [Google Scholar] [CrossRef]
- Lancelot, E.; Beal, M.F. Glutamate toxicity in chronic neurodegenerative disease. Prog. Brain Res. 1998, 116, 331–347. [Google Scholar] [PubMed]
- Orgogozo, J.M.; Rigaud, A.S.; Stoffler, A.; Mobius, H.J.; Forette, F. Efficacy and safety of memantine in patients with mild to moderate vascular dementia: A randomized, placebo-controlled trial (MMM 300). Stroke 2002, 33, 1834–1839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcock, G.; Mobius, H.J.; Stoffler, A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int. Clin. Psychopharmacol. 2002, 17, 297–305. [Google Scholar] [CrossRef]
- Folch, J.; Busquets, O.; Ettcheto, M.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Garcia, M.L.; Olloquequi, J.; Casadesús, G.; Beas-Zarate, C.; et al. Memantine for the treatment of dementia: A review on its current and future applications. J. Alzheim. Dis. 2018, 62, 1223–1240. [Google Scholar] [CrossRef] [Green Version]
- Matsunaga, S.; Kishi, T.; Iwata, N. Memantine monotherapy for Alzheimer’s disease: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0123289. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Hofer, E.; Bouwman, F.H.; Buerger, K.; Cordonnier, C.; Fladby, T.; Galimberti, D.; Georges, J.; Heneka, M.T.; Hort, J.; et al. EFNS-ENS/EAN guideline on concomitant use of cholinesterase inhibitors and memantine in moderate to severe Alzheimer’s disease. Eur. J. Neurol. 2015, 22, 889–898. [Google Scholar] [CrossRef]
- Winkler, F. The brain metastatic niche. J. Mol. Med. (Berl) 2015, 93, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Duman, J.G.; Dinh, J.; Zhou, W.; Cham, H.; Mavratsas, V.C.; Paveškovic, M.; Mulherkar, S.; McGovern, S.L.; Tolias, K.F.; Grosshans, D.R. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro-Oncol. 2018, 20, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Shirai, K.; Mizui, T.; Suzuki, Y.; Okamoto, M.; Hanamura, K.; Yoshida, Y.; Hino, M.; Noda, S.; Al-jahdari, W.S.; Chakravarti, A.; et al. X irradiation changes dendritic spine morphology and density through reduction of cytoskeletal proteins in mature neurons. Radiat. Res. 2013, 179, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef] [Green Version]
- Franchino, F.; Rudà, R.; Soffietti, R. Mechanisms and Therapy for Cancer Metastasis to the Brain. Front. Oncol. 2018, 8, 161. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, Y.R.; Li, X.A.; el Naqa, I.; Hahn, C.A.; Marks, L.B.; Merchant, T.E.; Dicker, A.P. Radiation dose-volume effects in the brain. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S20–S27. [Google Scholar] [CrossRef] [Green Version]
- Bentzen, S.M.; Constine, L.S.; Deasy, J.O.; Eisbruch, A.; Jackson, A.; Marks, L.B.; Ten Haken, R.K.; Yorke, E.D. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): An introduction to the scientific issues. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S3–S9. [Google Scholar] [CrossRef] [Green Version]
- Van der Maazen, R.W.M.; Kleiboer, B.J.; Verhagen, I.; van der Kogel, A.J. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. Int. J. Radiat. Biol. 1993, 63, 661–666. [Google Scholar] [CrossRef]
- Correa, D.D.; DeAngelis, L.M.; Shi, W.; Thaler, H.; Glass, A.; Abrey, L.E. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 2004, 62, 548–555. [Google Scholar] [CrossRef]
- Mulhern, R.K.; Merchant, T.E.; Gajjar, A.; Reddick, W.E.; Kun, L.E. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004, 5, 399–408. [Google Scholar] [CrossRef]
- Reddick, W.E.; Shan, Z.Y.; Glass, J.O.; Helton, S.; Xiong, X.; Wu, S.; Bonner, M.J.; Howard, S.C.; Christensen, R.; Khan, R.B.; et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer 2006, 106, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Anscher, M.S.; Swift, P.S.; Gaspar, L.E.; Marks, L.B. Radiation injury of the brain and spinal cord. In Neurosurgery; Wilkins, R.H., Rengachary, S.S., Eds.; McGraw-Hill: New York, NY, USA, 1996; pp. 1921–1936. [Google Scholar]
- Clarke, D.D.; Sokoloff, L. Circulation and energy metabolism of the brain. In Basic Neurochemistry: Molecular, Cellular, and Medical Aspects; Lippincott-Raven Publishers: Philadelphia, PA, USA, 1999; pp. 637–669. [Google Scholar]
- Robbins, M.E.; Zhao, W. Chronic oxidative stress and radiation-induced late normal tissue injury: A review. Int. J. Radiat. Biol. 2004, 80, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, A.M.; Leyrer, C.M.; Greene-Schloesser, D.M.; Shing, E.; Kearns, W.T.; Hinson, W.H.; Tatter, S.B.; Ip, E.H.; Rapp, S.R.; Robbins, M.E.; et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology 2013, 80, 747–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and functional implications of adult neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.E.; Patronas, N.; Hayes, W.; Grayson, J.; Becker, B.; Gnepp, D.; Rowland, J.; Anderson, A.; Glatstein, E.; Ihde, D.C.; et al. Neurologic, computed cranial tomographic, and magnetic resonance imaging abnormalities in patients with small-cell lung cancer: Further follow-up of 6- to 13- year survivors. J. Clin. Oncol. 1990, 8, 48–56. [Google Scholar] [CrossRef]
- Constine, L.S.; Konski, A.; Ekholm, S.; McDonald, S.; Rubin, P. Adverse effects of brain irradiation correlated with MR and CT imaging. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 319–330. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Stephens, L.C. Invited review: Permanent radiation myelopathy. Br. J. Radiol. 1992, 65, 737–753. [Google Scholar] [CrossRef]
- Stokes, T.B.; Niranjan, A.; Kano, H.; Choi, P.A.; Kondziolka, D.; Lunsford, L.D.; Monaco, E.A., 3rd. White matter changes in breast cancer brain metastases patients who undergo radiosurgery alone compared to whole brain radiation therapy plus radiosurgery. J. Neurooncol. 2015, 121, 583–590. [Google Scholar] [CrossRef]
- Patel, K.R.; Prabhu, R.S.; Kandula, S.; Oliver, D.E.; Kim, S.; Hadjipanayis, C.; Olson, J.J.; Oyesiku, N.; Curran, W.J.; Khan, M.K.; et al. Intracranial control and radiographic changes with adjuvant radiation therapy for resected brain metastases: Whole brain radiotherapy versus stereotactic radiosurgery alone. J. Neurooncol. 2014, 120, 657–663. [Google Scholar] [CrossRef]
- Trifiletti, D.M.; Lee, C.C.; Schlesinger, D.; Larner, J.M.; Xu, Z.; Sheehan, J.P. Leukoencephalopathy after stereotactic radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 870–878. [Google Scholar] [CrossRef]
- Tofilon, P.; Fike, J. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000, 153, 357–370. [Google Scholar] [CrossRef]
- Sheline, G.E.; Wara, W.M.; Smith, V. Therapeutic irradiation and brain injury. Int. J. Radiat. Oncol. Biol. Phys. 1980, 6, 1215–1228. [Google Scholar] [CrossRef]
- Armstrong, C.; Ruffer, J.; Corn, B.; DeVries, K.; Mollman, J. Biphasic patterns of memory deficits following moderate-dose partial-brain irradiation: Neuropsychologic outcome and proposed mechanisms. J. Clin. Oncol. 1995, 13, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Cross, N.E.; Glantz, M.J. Neurologic complications of radiation therapy. Neurol. Clin. 2003, 21, 249–277. [Google Scholar] [CrossRef]
- Evans, M.L.; Graham, M.M.; Mahler, P.A.; Rasey, J.S. Use of steroids to suppress vascular response to radiation. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 563–567. [Google Scholar] [CrossRef]
- Arvold, N.D.; Pinnell, N.E.; Mahadevan, A.; Connelly, S.; Silverman, R.; Weiss, S.E.; Kelly, P.J.; Alexander, B.M. Steroid and anticonvulsant prophylaxis for stereotactic radiosurgery: Large variation in physician recommendations. Pr. Radiat. Oncol. 2016, 6, e89–e96. [Google Scholar] [CrossRef]
- Helson, L. Radiation-induced Demyelination and Remyelination in the Central Nervous System: A Literature Review. Anticancer Res. 2018, 38, 4999–5002. [Google Scholar] [CrossRef] [Green Version]
- Nagesh, V.; Tsien, C.I.; Chenevert, T.L.; Ross, B.D.; Lawrence, T.S.; Junick, L.; Cao, Y. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: A diffusion tensor imaging study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1002–1010. [Google Scholar] [CrossRef] [Green Version]
- Rueß, D.; Pöhlmann, L.; Grau, S.; Hamisch, C.; Hellerbach, A.; Treuer, H.; Kocher, M.; Ruge, M.I. Long-term follow-up after stereotactic radiosurgery of intracanalicular acoustic neurinoma. Radiat. Oncol. 2017, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Faithfull, S.; Brada, M. Somnolence syndrome in adults following cranial irradiation for primary brain tumours. Clin. Oncol. 1998, 10, 250–254. [Google Scholar] [CrossRef]
- Curt, G.A.; Breitbart, W.; Cella, D.; Groopman, J.E.; Horning, S.J.; Itri, L.M.; Johnson, D.H.; Miaskowski, C.; Scherr, S.L.; Portenoy, R.K.; et al. Impact of cancer-related fatigue on the lives of patients: New findings from the Fatigue Coalition. Oncologist 2000, 5, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dy, S.M.; Lorenz, K.A.; Naeim, A.; Sanati, H.; Walling, A.; Asch, S.M. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J. Clin. Oncol. 2008, 26, 3886–3895. [Google Scholar] [CrossRef]
- Powell, C.; Guerrero, D.; Sardell, S.; Cumins, S.; Wharram, B.; Traish, D.; Gonsalves, A.; Ashley, S.; Brada, M. Somnolence syndrome in patients receiving radical radiotherapy for primary brain tumours: A prospective study. Radiother. Oncol. 2011, 100, 131–136. [Google Scholar] [CrossRef]
- Wilke, C.; Grosshans, D.; Duman, J.; Brown, P.; Li, J. Radiation-induced cognitive toxicity: Pathophysiology and interventions to reduce toxicity in adults. Neuro-Oncol. 2018, 20, 597–607. [Google Scholar] [CrossRef]
- Fink, J.; Born, D.; Chamberlain, M.C. Radiation necrosis: Relevance with respect to treatment of primary and secondary brain tumors. Curr. Neurol. Neurosci. Rep. 2012, 12, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Crossen, J.R.; Garwood, D.; Glatstein, E.; Neuwelt, E.A. Neurobehavioral sequelae of cranial irradiation in adults: A review of radiation-induced encephalopathy. J. Clin. Oncol. 1994, 12, 627–642. [Google Scholar] [CrossRef]
- DeAngelis, L.M.; Delattre, J.Y.; Posner, J.B. Radiation-induced dementia in patients cured of brain metastases. Neurology 1989, 39, 789–796. [Google Scholar] [CrossRef]
- Taphoorn, M.J.B.; Klein, M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004, 3, 159–168. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Kemp, S.; Benito-León, J.; Reuber, M. The influence of cognitive impairment on health-related quality of life in neurological disease. Acta Neuropsychiatr. 2010, 22, 2–13. [Google Scholar] [CrossRef]
- Meyers, C.A.; Hess, K.R.; Yung, W.A.; Levin, V.A. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J. Clin. Oncol. 2000, 18, 646–650. [Google Scholar] [CrossRef]
- Gilroy, J. Basic Neurology, 3rd ed.; McGraw-Hill Health Professions Division: New York, NY, USA, 2000. [Google Scholar]
- Meyers, C.A.; Weitzner, M.A.; Valentine, A.D.; Levin, V.A. Methylphenidate therapy improves cognition, mood and function of brain tumor patients. J. Clin. Oncol. 1998, 16, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Welzel, G.; Fleckenstein, K.; Schaefer, J.; Hermann, B.; Kraus-Tiefenbacher, U.; Mai, S.K.; Wenz, F. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef]
- Gondi, V.; Paulus, R.; Bruner, D.W.; Meyers, C.A.; Gore, E.M.; Wolfson, A.; Werner-Wasik, M.; Sun, A.Y.; Choy, H.; Movsas, B. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: Pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 656–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robison, L.L.; Armstrong, G.T.; Boice, J.D.; Chow, E.J.; Davies, S.M.; Donaldson, S.S.; Green, D.M.; Hammond, S.; Meadows, A.T.; Mertens, A.C.; et al. The Childhood Cancer Survivor Study: A National Cancer Institute–supported resource for outcome and intervention research. J. Clin. Oncol. 2009, 27, 2308–2318. [Google Scholar] [CrossRef] [Green Version]
- Brière, M.E.; Scott, J.G.; McNall-Knapp, R.Y.; Adams, R.L. Cognitive outcome in pediatric brain tumor survivors: Delayed attention deficit at long-term follow-up. Pediatr. Blood Cancer 2008, 50, 337–340. [Google Scholar] [CrossRef]
- Weitzner, M.A.; Meyers, C.A.; Valentine, A.D. Methylphenidate in the treatment of neurobehavioral slowing associated with cancer and cancer treatment. J. Neuropsychiatry Clin. Neurosci. 1995, 7, 347–350. [Google Scholar]
- Attia, A.; Page, B.R.; Lesser, G.J.; Chan, M. Treatment of radiation-induced cognitive decline. Curr. Treat. Options Oncol. 2014, 15, 539–550. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Kwong, D.L.W.; Leung, S.F.; Tung, S.Y.; Sze, W.M.; Sham, J.S.T.; Teo, P.M.L.; Leung, T.W.; Wu, P.M.; Chappell, R.; et al. Factors affecting risk of symptomatic temporal lobe necrosis: Significance of fractional dose and treatment time. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 75–85. [Google Scholar] [CrossRef]
- Szerlip, N.; Rutter, C.; Ram, N.; Yovino, S.; Kwok, Y.; Maggio, W.; Regine, W.F. Factors impacting volumetric white matter changes following whole brain radiation therapy. J. Neuro-Oncol. 2011, 103, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Vigliani, M.C.; Sichez, N.; Poisson, M.; Delatrre, J.Y. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4-year results. Int. J. Radiat. Oncol. Biol. Phys. 1996, 35, 527–533. [Google Scholar] [CrossRef]
- Lidstone, V.; Butters, E.; Seed, P.T.; Sinnott, C.; Beynon, T.; Richards, M. Symptoms and concerns amongst cancer outpatients: Identifying the need for specialist palliative care. Palliat. Med. 2003, 17, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Mukand, J.A.; Blackinton, D.D.; Crincoli, M.G.; Lee, J.J.; Santos, B.B. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. Am. J. Phys. Med. Rehabil. 2001, 80, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Meador, K.J.; Loring, D.W.; Abney, O.L.; Allen, M.E.; Moore, E.E.; Zamrini, E.Y.; King, D.W. Effects of carbamazepine and phenytoin on EEG and memory in healthy adults. Epilepsia 1993, 34, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Meyers, C.A.; Abbruzzese, J.L. Cognitive functioning in cancer patients: Effect of previous treatment. Neurology 1992, 42, 434–436. [Google Scholar] [CrossRef]
- Komaki, R.; Meyers, C.A.; Shin, D.M.; Garden, A.S.; Byrne, K.; Nickens, J.A.; Cox, J.D. Evaluation of cognitive function in patients with limited small cell lung cancer prior to and shortly following prophylactic cranial irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1995, 33, 179–182. [Google Scholar] [CrossRef]
- Brown, E.S. Effects of glucocorticoids on mood, memory, and the hippocampus. Treatment and preventive therapy. Ann. N. Y. Acad. Sci. 2009, 1179, 41–55. [Google Scholar] [CrossRef]
- Brown, E.S.; J Woolston, D.; Frol, A.; Bobadilla, L.; Khan, D.A.; Hanczyc, M.; Rush, A.J.; Fleckenstein, J.; Babcock, E.; Cullum, C.M. Hippocampal volume, spectroscopy, cognition, and mood in patients receiving corticosteroid therapy. Biol. Psychiatry 2004, 55, 538–545. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfılieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Monje, M.L.; Mizumatsu, S.; Fike, J.R.; Palmer, T.D. Irradiation induces neural precursor-cell dysfunction. Nat. Med. 2002, 8, 955–962. [Google Scholar] [CrossRef]
- Greene-Schloesser, D.; Robbins, M.E.; Peiffer, A.M.; Shaw, E.G.; Wheeler, K.T.; Chan, M.D. Radiation-induced brain injury: A review. Front. Oncol. 2012, 2, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Zhou, W.; Lam, T.T.; Weng, C.; Bronk, L.; Ma, D.; Wang, Q.; Duman, J.G.; Dougherty, P.M.; Grosshans, D.R. Radiation induces age-dependent deficits in cortical synaptic plasticity. Neuro-Oncol. 2018, 20, 1207–1214. [Google Scholar] [CrossRef]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs. stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef]
- Brown, P.D.; Jaeckle, K.; Ballman, K.V.; Farace, E.; Cerhan, J.H.; Anderson, S.K.; Carrero, X.W.; Barker, F.G., 2nd; Deming, R.; Burri, S.H.; et al. Effect of Radiosurgery Alone vs. Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016, 316, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wujanto, C.; Vellayappan, B.; Chang, E.L.; Chao, S.T.; Sahgal, A.; Lo, S.S. Radiotherapy to the brain: What are the consequences of this age-old treatment? Ann. Palliat. Med. 2021, 10, 936–952. [Google Scholar] [CrossRef] [PubMed]
- Padovani, L.; Andre, N.; Constine, L.S.; Muracciole, X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat. Rev. Neurol. 2012, 8, 578–588. [Google Scholar] [CrossRef]
- Wolfson, A.H.; Bae, K.; Komaki, R.; Meyers, C.; Movsas, B.; Le Pechoux, C.; Werner-Wasik, M.; Videtic, G.M.; Garces, Y.I.; Choy, H. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 77–84. [Google Scholar]
- Slotman, B.J.; Mauer, M.E.; Bottomley, A.; Faivre-Finn, C.; Kramer, G.W.; Rankin, E.M.; Snee, M.; Hatton, M.; Postmus, P.E.; Collette, L.; et al. Prophylactic cranial irradiation in extensive disease small-cell lung cancer: Short-term health-related quality of life and patient reported symptoms: Results of an international Phase III randomized controlled trial by the EORTC Radiation Oncology and Lung Cancer Groups. J. Clin. Oncol. 2009, 27, 78–84. [Google Scholar]
- Le Pechoux, C.; Laplanche, A.; Faivre-Finn, C.; Ciuleanu, T.; Wanders, R.; Lerouge, D.; Keus, R.; Hatton, M.; Videtic, G.M.; Senan, S.; et al. Clinical neurological outcome and quality of life among patients with limited small-cell cancer treated with two different doses of prophylactic cranial irradiation in the intergroup phase III trial (PCI99-01, EORTC 22003-08004, RTOG 0212 and IFCT 99-01). Ann. Oncol. 2011, 22, 1154–1163. [Google Scholar] [CrossRef]
- Sun, A.; Bae, K.; Gore, E.M.; Movsas, B.; Wong, S.J.; Meyers, C.A.; Bonner, J.A.; Schild, S.E.; Gaspar, L.E.; Bogart, J.A.; et al. Phase III trial of prophylactic cranial irradiation compared with observation in patients with locally advanced non-small-cell lung cancer: Neurocognitive and quality-of-life analysis. J. Clin. Oncol. 2011, 29, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Belka, C.; Budach, W.; Kortmann, R.D.; Bamberg, M. Radiation induced CNS toxicity: Molecular and cellular mechanisms. Br. J. Cancer 2001, 85, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Khasraw, M.; Posner, J.B. Neurological complications of systemic cancer. Lancet Neurol. 2010, 9, 1214–1227. [Google Scholar] [CrossRef]
- Shaw, E.G.; Rosdhal, R.; D’Agostino, R.B., Jr.; Lovato, J.; Naughton, M.J.; Robbins, M.E.; Rapp, S.R. Phase II study of donepezil in irradiated brain tumor patients: Effect on cognitive function, mood, and quality of life. J. Clin. Oncol. 2006, 24, 1415–1420. [Google Scholar] [CrossRef]
- Barani, I.J.; Larson, D.A.; Berger, M.S. Future directions in treatment of brain metastases. Surg. Neurol. Int. 2013, 4, S220–S230. [Google Scholar] [CrossRef]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro-Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Winblad, B.; Jones, R.W.; Wirth, Y.; Stöffler, A.; Möbius, H.J. Memantine in moderate to severe Alzheimer’s disease: A meta-analysis of randomized clinical trials. Dement. Geriatr. Cogn. Disord. 2007, 24, 20–27. [Google Scholar] [CrossRef]
- Chilukuri, S.; Burela, N. Memantine for Prevention of Brain Irradiation-Induced Cognitive Toxicity: A Tale of an Underappreciated and Underused Intervention. JCO Glob. Oncol. 2020, 6, 1384–1388. [Google Scholar] [CrossRef]
- Barnes, S.R.; Ng, T.S.; Montagne, A.; Law, M.; Zlokovic, B.V.; Jacobs, R.E. Optimal acquisition and modeling parameters for accurate assessment of low K blood-brain barrier permeability using dynamic contrast-enhanced MRI. Magn. Reson. Med. 2016, 75, 1967–1977. [Google Scholar] [CrossRef] [Green Version]
- Starr, J.M.; Farrall, A.J.; Armitage, P.; McGurn, B.; Wardlaw, J. Blood-brain barrier permeability in Alzheimer’s disease: A case-control MRI study. Psychiatry Res. 2009, 171, 232–241. [Google Scholar] [CrossRef]
- Wang, H.; Golob, E.J.; Su, M.Y. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J. Magn. Reson. Imaging 2006, 24, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Tsien, C.I.; Sundgren, P.C.; Nagesh, V.; Normolle, D.; Buchtel, H.; Junck, L.; Lawrence, T.S. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for prediction of radiation-induced neurocognitive dysfunction. Clin. Cancer Res. 2009, 15, 1747–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlachos, F.; Tung, Y.S.; Konofagou, E. Permeability dependence study of the focused ultrasound-induced bloodbrain barrier opening at distinct pressures and microbubble diameters using DCE-MRI. Magn. Reson. Med. 2011, 66, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.; Leppert, I.R.; Roberge, D.; Boudam, K.; Brown, P.D.; Muanza, T.; Pike, G.B.; Chankowsky, J.; Mihalcioiu, C. A pilot study using dynamic contrast enhanced-MRI as a response biomarker of the radioprotective effect of memantine in patients receiving whole brain radiotherapy. Oncotarget 2016, 7, 50986–50996. [Google Scholar] [CrossRef] [Green Version]
- Laack, N.N.; Pugh, S.L.; Brown, P.D.; Fox, S.; Wefel, J.S.; Meyers, C.; Choucair, A.; Khuntia, D.; Suh, J.H.; Roberge, D.; et al. The association of health-related quality of life and cognitive function in patients receiving memantine for the prevention of cognitive dysfunction during whole-brain radiotherapy. Neuro-Oncol. Pract. 2019, 6, 274–282. [Google Scholar] [CrossRef]
- Tsai, P.F.; Yang, C.C.; Chuang, C.C.; Huang, T.Y.; Wu, Y.M.; Pai, P.C.; Tseng, C.K.; Wu, T.H.; Shen, Y.L.; Lin, S.Y. Hippocampal dosimetry correlates with the change in neurocognitive function after hippocampal sparing during whole brain radiotherapy: A prospective study. Radiat. Oncol. 2015, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez, A.N.; Westerly, D.C.; Tomé, W.A.; Jaradat, H.A.; Mackie, T.R.; Bentzen, S.M.; Khuntia, D.; Mehta, M.P. Whole brain radiotherapy with hippocampal avoidance and simultaneously integrated brain metastases boost: A planning study. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 589–597. [Google Scholar] [CrossRef] [Green Version]
- Gondi, V.; Tolakanahalli, R.; Mehta, M.P.; Tewatia, D.; Rowley, H.; Kuo, J.S.; Khuntia, D.; Tomé, W.A. Hippocampal-sparing whole-brain radiotherapy: A “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1244–1252. [Google Scholar] [CrossRef] [Green Version]
- Ghia, A.; Tomé, W.A.; Thomas, S.; Cannon, G.; Khuntia, D.; Kuo, J.S.; Mehta, M.P. Distribution of brain Metastases in relation to the Hippocampus: Implications for neurocognitive functional preservation. Int. J. Radiat. Oncology. 2007, 68, 971–977. [Google Scholar] [CrossRef]
- Barani, I.J.; Benedict, S.H.; Lin, P.S. Neural stem cells: Implications for the conventional radiotherapy of central nervous system malignancies. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 324–333. [Google Scholar] [CrossRef]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase III Trial NRG Oncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Andratschke, N.; Belderbos, J.; Mayinger, M.; Schagen, S.B.; De Ruysscher, D. Hippocampal avoidance and memantine for whole-brain radiotherapy: Long-term follow-up warranted. J. Clin. Oncol. 2020, 38, 3454–3455. [Google Scholar] [CrossRef]
- Levy, A.; Dhermain, F.; Botticella, A.; Rivera, S.; Le Péchoux, C. Hippocampal avoidance whole-brain radiotherapy (WBRT) Versus WBRT in patients with brain metastases: Were hippocampi the only difference? J. Clin. Oncol. 2020, 38, 3453–3454. [Google Scholar] [CrossRef]
- Krayenbuehl, J.; Di Martino, M.; Guckenberger, M.; Andratschke, N. Improved plan quality with automated radiotherapy planning for whole brain with hippocampus sparing: A comparison to the RTOG 0933 trial. Radiat. Oncol. 2017, 12, 161, Erratum in Radiat. Oncol. 2017, 12, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperduto, P.W.; Yang, T.J.; Beal, K.; Pan, H.; Brown, P.D.; Bangdiwala, A.; Shanley, R.; Yeh, N.; Gaspar, L.E.; Braunstein, S.; et al. Estimating survival in patients with lung cancer and brain metastases: An update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA). JAMA Oncol. 2017, 3, 827–831. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Kased, N.; Roberge, D.; Xu, Z.; Shanley, R.; Luo, X.; Sneed, P.K.; Chao, S.T.; Weil, R.J.; Suh, J.; et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. 2012, 30, 419–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schimmel, W.C.M.; Gehring, K.; Hanssens, P.E.J.; Sitskoorn, M.M. Cognitive functioning and predictors thereof in patients with 1-10 brain metastases selected for stereotactic radiosurgery. J. Neurooncol. 2019, 145, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. A multi-institutional prospective observational study of stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901 study update): Irradiation-related complications and long-term maintenance of MiniMental State Examination Scores. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Le Péchoux, C.; Sun, A.; Slotman, B.J.; De Ruysscher, D.; Belderbos, J.; Gore, E.M. Prophylactic cranial irradiation for patients with lung cancer. Lancet Oncol. 2016, 17, e277–e293. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J. Clin. Oncol. 2017, 35, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Chun, S.G.; Klopp, A.H. Meeting the burden of proof to justify intensity-modulated radiation for brain metastases. J. Clin. Oncol. 2020, 38, 3452. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Brown, P.D. Reply to S.G. Chun et al, A. Levy et al, and N. Andratschke et al. J. Clin Oncol. 2020, 38, 3455–3457. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Pugh, S.; Brown, P.D.; Wefel, J.S.; Tome, W.A.; Armstrong, T.S.; Bruner, D.W.; Bovi, J.A.; Robinson, C.G.; Khuntia, D.; et al. Significant preservation of neurocognitive function (NCF) and patient-reported symptoms with hippocampal avoidance (HA) during whole-brain radiotherapy (WBRT) for brain metastases: Final results of NRG Oncology CC001. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, S12–S13. [Google Scholar] [CrossRef]
- Armstrong, T.; Deshmukh, S.; Brown, P.D.; Gondi, V.; Benzinger, T.; Gilbert, M.; Tome, W.; Wefel, J.; Bruner, D.; Roberge, D.; et al. Significant preservation of neurocognitive function and patient-reported symptoms with hippocampal avoidance during whole-brain radiotherapy for brain metastases: Longterm results of NRG Oncology CC001. Neuro-Oncol. 2019, 21, 24–25. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network: Central Nervous System Cancers. 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx (accessed on 25 May 2021).
- NICE Guideline. Brain Tumours (Primary) and Brain Metastases in Adults; NICE: London, UK, 2018. [Google Scholar]
- Lamba, N.; Mehanna, E.; Kearney, R.B.; Catalano, P.J.; Brown, P.D.; Haas-Kogan, D.A.; Aizer, A.A. Prescription of memantine during non-stereotactic, brain-directed radiation among patients with brain metastases: A population-based study. J. Neurooncol. 2020, 148, 509–517. [Google Scholar] [CrossRef]
- Mitchell, D.K.; Kwon, H.J.; Kubica, P.A.; Huff, W.X.; O’Regan, R.; Dey, M. Brain metastases: An update on the multi-disciplinary approach of clinical management. Neurochirurgie 2022, 68, 69–85. [Google Scholar] [CrossRef]
- Barbour, A.B.; Jacobs, C.D.; Williamson, H.; Floyd, S.R.; Suneja, G.; Torok, J.A.; Kirkpatrick, J.P. Radiation therapy practice patterns for brain metastases in the United States in the stereotactic radiosurgery era. Adv. Radiat. Oncol. 2019, 5, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Farrell, M.J.; Yahya, J.B.; Degnin, C.; Chen, Y.; Holland, J.M.; Henderson, M.A.; Jaboin, J.J.; Harkenrider, M.M.; Thomas, C.R., Jr.; Mitin, T. Prophylactic Cranial Irradiation for Limited-Stage Small-Cell Lung Cancer: Survey of US Radiation Oncologists on Current Practice Patterns. Clin. Lung Cancer 2018, 19, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yamanaka, T.; Seto, T.; Harada, H.; Nokihara, H.; Saka, H.; Nishio, M.; Kaneda, H.; Takayama, K.; Ishimoto, O.; et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017, 18, 663–671. [Google Scholar] [CrossRef]
- Slotman, B.; Faivre-Finn, C.; Kramer, G.; Rankin, E.; Snee, M.; Hatton, M.; Postmus, P.; Collette, L.; Musat, E.; Senan, S.; et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N. Engl. J. Med. 2007, 357, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Aupérin, A.; Arriagada, R.; Pignon, J.P.; Le Péchoux, C.; Gregor, A.; Stephens, R.J.; Kristjansen, P.E.; Johnson, B.E.; Ueoka, H.; Wagner, H.; et al. Prophylactic cranial irradiation for patients with small-cell lung cancer in complete remission. Prophylactic Cranial Irradiation Overview Collaborative Group. N. Engl. J. Med. 1999, 341, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Kalemkerian, G.P.; Akerley, W.; Bogner, P.; Borghaei, H.; Chow, L.Q.; Downey, R.J.; Gandhi, L.; Ganti, A.K.P.; Govindan, R.; Grecula, J.C.; et al. Small cell lung cancer. J. Natl. Compr. Canc. Netw. 2013, 11, 78–98. [Google Scholar] [CrossRef] [Green Version]
- Rusthoven, C.G.; Kavanagh, B.D. Prophylactic cranial irradiation (PCI) versus active MRI surveillance for small cell lung cancer: The case for equipoise. J. Thorac. Oncol. 2017, 12, 1746–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robin, T.P.; Jones, B.L.; Amini, A.; Koshy, M.; Gaspar, L.E.; Liu, A.K.; Nath, S.K.; Kavanagh, B.D.; Camidge, D.R.; Rusthoven, C.G. Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer 2018, 120, 88–90. [Google Scholar] [CrossRef]
- Cifarelli, C.P.; Vargo, J.A.; Fang, W.; Liscak, R.; Guseynova, K.; Warnick, R.E.; Lee, C.C.; Yang, H.C.; Borghei-Razavi, H.; Maiti, T.; et al. Role of Gamma Knife Radiosurgery in Small Cell Lung Cancer: A Multi- Institutional Retrospective Study of the International Radiosurgery Research Foundation (IRRF). Neurosurgery 2020, 87, 664–671. [Google Scholar] [CrossRef]
- Rusthoven, C.G. Small Cell Lung Cancer: PCI Uncertainty and Emerging Radiosurgery Interest. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1034–1035. [Google Scholar] [CrossRef]
- Taylor, J.M.; Rusthoven, C.G.; Moghanaki, D. Prophylactic cranial irradiation or MRI surveillance for extensive stage small cell lung cancer. J. Thorac. Dis. 2020, 12, 6225–6233. [Google Scholar] [CrossRef]
- Yoon, W.S.; Yeom, M.Y.; Kang, E.S.; Chung, Y.A.; Chung, D.S.; Jeun, S.S. Memantine Induces NMDAR1-Mediated Autophagic Cell Death in Malignant Glioma Cells. J. Korean Neurosurg. Soc. 2017, 60, 130–137. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Sun, B.; Wu, Z.; Guo, Q.; Guo, Y. In vivo single-voxel proton MR spectroscopy in the differentiation of high-grade gliomas and solitary metastases. Clin. Radiol. 2004, 59, 77–85. [Google Scholar] [CrossRef]
- Rijpkema, M.; Schuuring, J.; van der Meulen, Y.; van der Graaf, M.; Bernsen, H.; Boerman, R.; van der Kogel, A.; Heerschap, A. Characterization of oligodendrogliomas using short echo time 1H MR spectroscopic imaging. NMR Biomed. 2003, 16, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.; Khalili, K. Molecular mechanisms of necrosis in glioblastoma: The role of glutamate excitotoxicity. Cancer Biol. Ther. 2009, 8, 1791–1797. [Google Scholar] [CrossRef]
- Ramaswamy, P.; Aditi Devi, N.; Hurmath Fathima, K.; Dalavaikodihalli Nanjaiah, N. Activation of NMDA receptor of glutamate influences MMP-2 activity and proliferation of glioma cells. Neurol. Sci. 2014, 35, 823–829. [Google Scholar] [CrossRef]
- Ishiuchi, S.; Yoshida, Y.; Sugawara, K.; Aihara, M.; Ohtani, T.; Watanabe, T.; Saito, N.; Tsuzuki, K.; Okado, H.; Miwa, A.; et al. Ca2+-permeable AMPA receptors regulate growth of human glioblastoma via Akt activation. J. Neurosci. 2007, 27, 7987–8001. [Google Scholar] [CrossRef] [Green Version]
- Stepulak, A.; Luksch, H.; Gebhardt, C.; Uckermann, O.; Marzahn, J.; Sifringer, M.; Rzeski, W.; Staufner, C.; Brocke, K.S.; Turski, L.; et al. Expression of glutamate receptor subunits in human cancers. Histochem. Cell Biol. 2009, 132, 435–445. [Google Scholar] [CrossRef]
- Rzeski, W.; Turski, L.; Ikonomidou, C. Glutamate antagonists limit tumor growth. Proc. Natl. Acad. Sci. USA 2001, 98, 6372–6377. [Google Scholar] [CrossRef] [Green Version]
- Takano, T.; Lin, J.H.; Arcuino, G.; Gao, Q.; Yang, J.; Nedergaard, M. Glutamate release promotes growth of malignant gliomas. Nat. Med. 2001, 7, 1010–1015. [Google Scholar] [CrossRef]
- Müller-Längle, A.; Lutz, H.; Hehlgans, S.; Rödel, F.; Rau, K.; Laube, B. NMDA Receptor Mediated Signaling Pathways Enhance Radiation Resistance, Survival and Migration in Glioblastoma Cells-A Potential Target for Adjuvant Radiotherapy. Cancers (Basel) 2019, 11, 503. [Google Scholar] [CrossRef] [Green Version]
- Cacciatore, I.; Fornasari, E.; Marinelli, L.; Eusepi, P.; Ciulla, M.; Ozdemir, O.; Tatar, A.; Turkez, H.; Di Stefano, A. Memantine-derived drugs as potential antitumor agents for the treatment of glioblastoma. Eur. J. Pharm. Sci. 2017, 109, 402–411. [Google Scholar] [CrossRef]
- Altinoz, M.A.; Elmaci, İ. Targeting nitric oxide and NMDA receptor-associated pathways in treatment of high grade glial tumors. Hypotheses for nitro-memantine and nitrones. Nitric Oxide 2018, 79, 68–83. [Google Scholar] [CrossRef]
- Maraka, S.; Groves, M.D.; Mammoser, A.G.; Melguizo-Gavilanes, I.; Conrad, C.A.; Tremont-Lukats, I.W.; Loghin, M.E.; O’Brien, B.J.; Puduvalli, V.K.; Sulman, E.P.; et al. Phase 1 lead-in to a phase 2 factorial study of temozolomide plus memantine, mefloquine, and metformin as postradiation adjuvant therapy for newly diagnosed glioblastoma. Cancer 2019, 125, 424–433, Erratum in Cancer 2019, 125, 1387. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Bentzen, S.M.; Renschler, M.; Mehta, M.P. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J. Clin. Oncol. 2007, 25, 1260–1266. [Google Scholar] [CrossRef]
- Brown, P.D.; Jensen, A.W.; Felten, S.J.; Ballman, K.V.; Schaefer, P.L.; Jaeckle, K.A.; Cerhan, J.H.; Buckner, J.C. Detrimental effects of tumor progression on cognitive function of patients with high-grade glioma. J. Clin. Oncol. 2006, 24, 5427–5433. [Google Scholar] [CrossRef]
- Nogueira, A.B.; Hoshino, H.S.R.; Ortega, N.C.; Dos Santos, B.G.S.; Teixeira, M.J. Adult human neurogenesis: Early studies clarify recent controversies and go further. Metab. Brain Dis. 2022, 37, 153–172. [Google Scholar] [CrossRef]
- Bernier, P.J.; Vinet, J.; Cossette, M.; Parent, A. Characterization of the subventricular zone of the adult human brain: Evidence for involvement of Bcl-2. Neurosci. Res. 2000, 37, 67–78. [Google Scholar] [CrossRef]
- Sharif, A.; Fitzsimons, C.P.; Lucassen, P.J. Neurogenesis in the adult hypothalamus: A distinct form of structural plasticity involved in metabolic and circadian regulation, with potential relevance for human pathophysiology. Handb. Clin. Neurol. 2021, 179, 125–140. [Google Scholar] [CrossRef]
- Dahshan, B.A.; Mattes, M.D.; Bhatia, S.; Palek, M.S.; Cifarelli, C.P.; Hack, J.D.; Vargo, J.A. Efficacy of Stereotactic Radiosurgery in Patients with Multiple Metastases: Importance of Volume Rather Than Number of Lesions. Cureus 2017, 9, e1966. [Google Scholar] [CrossRef] [Green Version]
- Handisurya, A.; Rumpold, T.; Caucig-Lütgendorf, C.; Flechl, B.; Preusser, M.; Ilhan-Mutlu, A.; Dieckmann, K.; Widhalm, G.; Grisold, A.; Wöhrer, A.; et al. Are hypothyroidism and hypogonadism clinically relevant in patients with malignant gliomas? A longitudinal trial in patients with glioma. Radiother. Oncol. 2019, 130, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2010, 205, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Fahlbusch, F.B.; Rades, D.; Schmid, S.M.; Gebauer, J.; Janssen, S. Are hypothalamic pituitary (HP) axis deficiencies after whole brain radiotherapy (WBRT) of relevance for adult cancer patients?—A systematic review of the literature. BMC Cancer 2019, 19, 1213. [Google Scholar] [CrossRef] [Green Version]
- Mehta, P.; Janssen, S.; Fahlbusch, F.B.; Schmid, S.M.; Gebauer, J.; Cremers, F.; Ziemann, C.; Tartz, M.; Rades, D. Sparing the hippocampus and the hypothalamic- pituitary region during whole brain radiotherapy: A volumetric modulated arc therapy planning study. BMC Cancer 2020, 20, 610. [Google Scholar] [CrossRef]
- Perez De Celis, E.S.; Li, D.; Sun, C.; Kim, H.; Twardowski, P.; Fakih, M.; Chung, V.; Cristea, M.; Lim, D.; Yuan, Y.; et al. Patient-defined goals and preferences among older patients with cancer starting chemotherapy (CT). J. Clin. Oncol. 2018, 36, 10009. [Google Scholar] [CrossRef]
- Shrestha, A.; Martin, C.; Burton, M.; Walters, S.; Collins, K.; Wyld, L. Quality of life versus length of life considerations in cancer patients: A systematic literature review. Psychooncology 2019, 28, 1367–1380. [Google Scholar] [CrossRef]
- Zheng, J.; Aljabab, S.; Lacasse, P.; Bahm, J.; Lekx-Toniolo, K.; Grimard, L. Functional cranio-spinal irradiation: A hippocampal and hypothalamic-pituitary axis sparing radiation technique using two IMRT modalities. Med. Dosim. 2020, 45, 190–196. [Google Scholar] [CrossRef]
- Janssen, S.; Mehta, P.; Bartscht, T.; Schmid, S.M.; Fahlbusch, F.B.; Rades, D. Prevalence of metastases within the hypothalamic-pituitary area in patients with brain metastases. Radiat. Oncol. 2019, 14, 152. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.W.; Wang, J.Q.; Wu, J.L.; Wang, H.B.; Wu, K.L. Simultaneously avoiding the hippocampus and hypothalamic-pituitary axis during whole brain radiotherapy: A planning study. Med. Dosim. 2019, 44, 130–135. [Google Scholar] [CrossRef]
- Li, J.; Ludmir, E.B.; Wang, Y.; Guha-Thakurta, N.; McAleer, M.F.; Settle, S.H.; Yeboa, D.N.; Ghia, A.J.; McGovern, S.L.; Chung, C.; et al. Stereotactic Radiosurgery versus Whole-brain Radiation Therapy for Patients with 4-15 Brain Metastases: A Phase III Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S21–S22. [Google Scholar] [CrossRef]
- Wagner, H.; Ali, A.; Glantz, M.; Blakeley, A. Role of Hippocampal-Avoidance Whole Brain Radiation Therapy (HA-WBRT) in Patients with Primary CNS Lymphoma (PCNSL). Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E424. [Google Scholar] [CrossRef]
- Pinkham, M.B.; Bertrand, K.C.; Olson, S.; Zarate, D.; Oram, J.; Pullar, A.; Foote, M.C. Hippocampal-sparing radiotherapy: The new standard of care for World Health Organization grade II and III gliomas? J. Clin. Neurosci. 2014, 21, 86–90. [Google Scholar] [CrossRef]
- Kim, K.S.; Wee, C.W.; Seok, J.Y.; Hong, J.W.; Chung, J.B.; Eom, K.Y.; Kim, J.S.; Kim, C.Y.; Park, Y.H.; Kim, Y.J.; et al. Hippocampus-sparing radiotherapy using volumetric modulated arc therapy (VMAT) to the primary brain tumor: The result of dosimetric study and neurocognitive function assessment. Radiat. Oncol. 2018, 13, 29. [Google Scholar] [CrossRef] [PubMed]
| Authors/Year [Ref] | Trial | Study Design | Study Characteristic | Main Findings |
|---|---|---|---|---|
| Brown PD et al., 2013 [129] | RTOG 0614 | Phase III, Random | Sample size and inclusion criteria: 508 adult patients with BM (only 149 evaluable at 24 weeks). Treatment: Patients were randomized to WBRT plus placebo versus WBRT plus memantine (20 mg/day for 24 weeks, starting within 3 days of WBRT start) | Similar grade 3–4 toxicity and study compliance in the two arms. Lower rate of decline in delayed recall (at 24 weeks) in the memantine arm but without reaching statistical significance (p: 0.059). Memantine arm: significantly longer time to CD (53.8% versus 64.9% at 24 weeks; HR: 0.78; 95% CI: 0.62–0.99, p: 0.01). better executive function at 24 weeks (p: 0.008 at 8 weeks; p: 0.0041 at 16 weeks), processing speed (p: 0.0137), and delayed recognition (p: 0.0149). |
| Laack NN et al., 2019 [138] | RTOG 0614 (subanalysis) | Phase III, Random | Subanalysis of the RTOG 0614 trial evaluating the correlation between health-related quality of life and cognitive function using FACT-Br and MOS-C | 149 patients completed FACT-Br, MOS-C, and objective cognitive assessments at 24 weeks. Over time: worsening in all domains of objective cognitive function with no differences in FACT-Br and MOS-C between the 2 arms. improvement of emotional and functional well-being (FACT) with stability of the other FACT-Br domains. Conversely, declined MOS-C scores. |
| Tsai PF et al., 2015 [139] | RTOG 0933 | Prospective | Sample size and inclusion criteria: 40 patients participated in an NCF assessment, including memory, executive function and psychomotor speed, before and after (4 months) HS-WBRT (assessments available in 24 patients). Treatment: therapeutic or prophylactic HS-WBRT. DVHs were generated for the left hippocampus, right hippocampus and hippocampal composite structure by calculating EQD2 (α/β: 2 Gy). | NCF scores are fairly stable before and after HS-WBRT in terms of hippocampus-dependent memory. EQD2 values < 12.60 Gy, <8.81 Gy, <7.45 Gy, and <5.83 Gy to 0%, 10%, 50%, and 80% volume of the hippocampal composite structure were significantly associated with preserved verbal memory. Specific dosimetric parameters of the left hippocampus impacted immediate recall of verbal memory (adjusted OR: 4.08; p: 0.042). |
| Brown PD et al., 2020 [145] | NRGCC001 | Phase III, Random | Sample size and inclusion criteria: 518 adult patients with BM Treatment: Patients underwent HS-WBRT plus memantine versus WBRT plus memantine. Primary endpoint: time to CD (defined as a decline in at least one of the cognitive tests). Secondary endpoints: OS, intracranial PFS, toxicity and patient-reported symptom burden. | HS-WBRT arm: significantly lower CD risk (adjusted HR: 0.74; 95% CI: 0.58–0.95; p: 0.02), due to the lesser impairment of learning and memory at 6 months (11.5% versus 24.7% [p: 0.049] and 16.4% versus 33.3% [p: 0.02], respectively) and executive function at 4 months (23.3% versus 40.4%; p: 0.01). no differences in terms of OS, intracranial PFS and toxicity. at 6 months: less difficulty speaking (p: 0.049), less memory deficits (p: 0.01), and less fatigue (p: 0.04). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scampoli, C.; Cammelli, S.; Galietta, E.; Siepe, G.; Buwenge, M.; Macchia, G.; Deodato, F.; Cilla, S.; Strigari, L.; Chiesa, S.; et al. Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review. Cancers 2022, 14, 2736. https://doi.org/10.3390/cancers14112736
Scampoli C, Cammelli S, Galietta E, Siepe G, Buwenge M, Macchia G, Deodato F, Cilla S, Strigari L, Chiesa S, et al. Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review. Cancers. 2022; 14(11):2736. https://doi.org/10.3390/cancers14112736
Chicago/Turabian StyleScampoli, Claudia, Silvia Cammelli, Erika Galietta, Giambattista Siepe, Milly Buwenge, Gabriella Macchia, Francesco Deodato, Savino Cilla, Lidia Strigari, Silvia Chiesa, and et al. 2022. "Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review" Cancers 14, no. 11: 2736. https://doi.org/10.3390/cancers14112736
APA StyleScampoli, C., Cammelli, S., Galietta, E., Siepe, G., Buwenge, M., Macchia, G., Deodato, F., Cilla, S., Strigari, L., Chiesa, S., & Morganti, A. G. (2022). Memantine in the Prevention of Radiation-Induced Brain Damage: A Narrative Review. Cancers, 14(11), 2736. https://doi.org/10.3390/cancers14112736







